DIGITAL,
PHARMACEUTICAL
BUSINESS
SUITE
COMPLETE PHARMACEUTICAL CLOUD-BASED SOLUTION

COMPLIANCE & GUIDELINES – USFDA || WHO || Cgmp || 21 CFR Part 11 eBMR with Production Management & QA/QC with COA or STP Management
Digital B-Line Suite
B-Line Suite: Transformative pharmaceutical and chemical enterprise software, ensuring seamless digitization, automation, and integration. Elevate productivity, quality, and efficiency while reducing costs through a system-driven approach, fostering business success.


Pharma Sales & Procure Control Management
Efficient management of pharmaceutical sales and procurement processes.

Pharma Warehouse Control Management – Barcode / RFID Control.
Streamlined pharmaceutical warehouse control with Barcode/RFID precision management.

Pharma Full Production Management & Operation with eBMR
Comprehensive pharmaceutical production management, seamlessly operated with electronic Batch Manufacturing Records (eBMR).

Pharma Doccell Management
Effective pharmaceutical document control and management for streamlined operations.

Pharma eQMS Management
Pharmaceutical Electronic Quality Management System (eQMS) for streamlined quality control.

Pharma eLog / eLog Sheet Management
Efficient pharmaceutical electronic log and log sheet management system.

Pharma eCalibration Management (Assets)
Optimized pharmaceutical electronic calibration management for precise asset control.

Pharma Diagnosys System
Advanced pharmaceutical diagnostics system for enhanced medical analysis and decision-making.

Annual Product Quality Review (APQR/PQR/APR)
Thorough Annual Product Quality Review (APQR/PQR/APR) for comprehensive quality assessment and continuous improvement.

Admin Master & User Access Management
Administrative control and user access management for streamlined system governance.

Pharma & IOT Board – IOT Devices Linkage with Assets.
Integration of pharmaceutical processes with IoT devices for enhanced asset linkage and management.
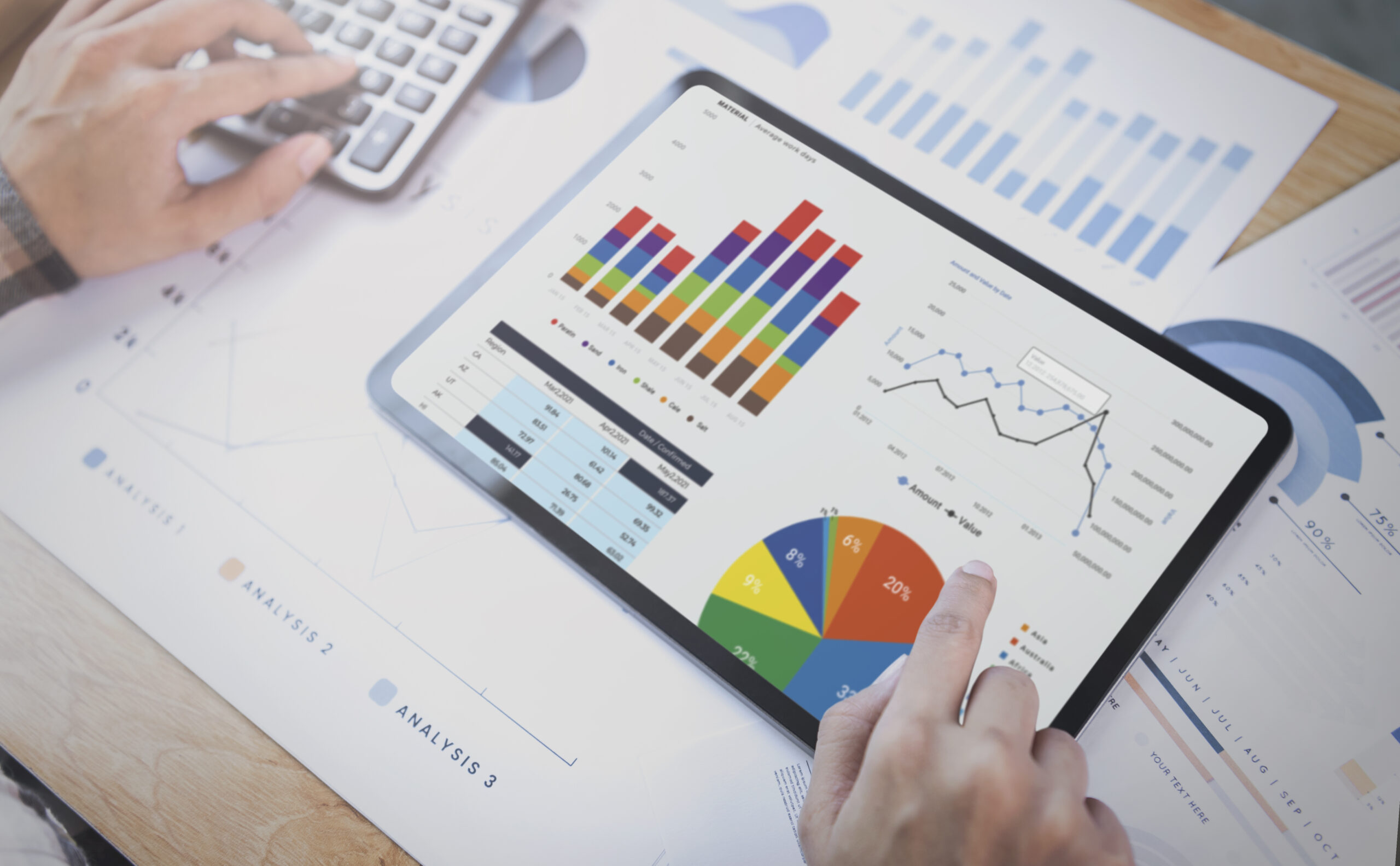
Business intelligence tool – MIS Builder & Report Analytics.
Dynamic business intelligence tool—MIS Builder & Report Analytics—for informed decision-making and strategic insights.

Audittrail, DB Auditlog, DB Health & Load Monitering, DB Admin
Robust auditing with Audit Trail, DB Audit Log, DB Health & Load Monitoring, and Database Administration for efficient database management.

Server Health & Performance Monitoring System
Comprehensive Server Health & Performance Monitoring System for optimal server functionality and performance.

Disaster Recovery & Backup Management tool
Resilient Disaster Recovery & Backup Management tool for safeguarding critical data and ensuring business continuity.

Pharma Sales & Procure Control Management
Presales Enquiry Management with sales Funnel
Efficient Presales Enquiry Management featuring a dynamic sales funnel for streamlined customer interactions and conversion tracking.
Sales Quote & Sales Order Management.
Seamless Sales Quote and Sales Order Management for efficient and organized sales processes.
Sale Export, Scrap, Revision Order Management.
Comprehensive Sales Export, Scrap, and Revision Order Management for versatile sales process control.
Blanket & Estimate Order
Streamlined Blanket and Estimate Order Management for flexible and efficient order processing.
Feedback & Complain Management
Effective Feedback & Complaint Management for responsive customer engagement and issue resolution.
Teams management - performance-wise, zone-wise, sector-wise and country-wise.
Strategic teams management for optimized performance, organized by zones, sectors, and countries.
Procure Request, Procure Order, Procure Indent, Import Order, Service PO, Blanket, Tendor Order
Efficient Procure Request, Order, Indent, Import Order, Service PO, Blanket, and Tender Order Management for comprehensive procurement control.
Vendor control & management
Systematic vendor control and management for streamlined and effective supplier relationships.
Complete procure management with form-formulation and checklist
End-to-end procurement management, featuring form formulation and checklist integration for comprehensive control and efficiency.
Advanced Matrix Report
Sophisticated Matrix Reporting for in-depth and advanced data analysis and visualization.
Pharma Warehouse Control Management
Material ISSUE – MIN, SMIN, DMIN, MMIN with Pharma Quality Control previous & new tests like Assay, moisture, Putity.
Material Batch, Lots, Expiration Dates with pharma specifications like moisture & assy formulas linkage
Stock Location & Wharehouse Control with FIFO, LIFO, FEFO.
Production Management, QC, eQMS, LIMS & Operation with eBMR:
- SOP, STP, MFR Master with Digital Approval Method with a digital sign and version control.
- RM, FG, PM, WFI, COA, Under Test Label, Approved Label generated with Digital Approval Method with digital sign.
- RM, FG, PM, WFI Specification with Digital Approval Method with a digital sign and version control.
- Filed level audit logs and User level permission with change control.
- Control Multi-Level of BOM – FG-RM-PM, BOM Versions control, Conversion Factor, Child BOM, Overage management, Dosageform and MFR control.
- Production Planning, Operation Planning, Material Planning, Resource Planning.
- Production Planning execution Shift wise, Operation wise.
- Batch control based on Dosage form – Dry Injection, Liquid Injection, Dry Powder, Capsule, Syrup, Dropper, Tablet.
- Batch control record with Start to Completion and single click generate eBMR Report with Digital Approval Method with digital sign.
- Batch record link with Bulk IPQC & Quality Control Specification with formulation.
- Auto % yield calculation.
- Auto Calculate Final accounability.
- Auto-calculate Batch code Detail.
- Control Operation with Deviation, User-level permission, Audit log, and change control.
- Advanced Matrix Report
- Module for electronic batch records that allows manufacturing records to be routed, reviewed, and approved.
- Scheduling batches in real-time to offer production plans.
- Electronic instructions are used to ensure that employees adhere to defined processes and procedures.
- Line clearance for all operation.
- Allows the software to be used by individuals who are both qualified and authorized.
- Exception handling module for detecting irregularities in manufacturing processes.
- Electronic signatures and audit trails are provided for all modules of the manufacturing process and system operation activities.
- Different levels of permission according to the role.
- Barcodes & Unique No can be used to trace the progression of an This eBMR Software will be able to generate the Barcodes with respective to Room, Equipment, material, and product.
- Throughout the procedure, user activity is logged with a date and time stamp.
- Product label management.
- Graphical reports on the latest trends and batch certificates.

Pharma Doccell Management

Document Template Management

Document Attachment & Indexion
Document Requisition and Print Control

Automatic Version Control

Revision History

Child Documents Management

Document Collaboration

Content Search

Dashboard & Report

Role-based Access

Advanced Matrix Report
- eLogs application will meet the compliance requirements with industry standards.
- E-Log Management System ensures to validate your data inputs, if there is incomplete log forms the system will not allow to submit it to the next process flow. It ensures integrity of data contained in eLogbook with all the data recorded as per your operations.
- Electronic Logbook Software (eLogbook) can create and design the log sheets (forms), as per the approved SOP’s, like equipment logs, calibration logs, Cleaning/ maintenance logs, production logs, and other usage logs.
- E-Log Management (eLog) Solution automatically manage multiple versions of a logbook with a proper audits available on few clicks, this eLog system does not allow you to access old version of logbooks once the new version is produced. Version control allows you to track all changes who made the changes to the log template and when it is made.
- E-Log Management Solution helps in mapping resources and equipment of a specific area of Users can cross check the availability of resources and the mapped equipment and instruments, etc. They can use the equipment and users accordingly during the production process in the facilities.
- Ability to generate custom reports based on user specified KPIs, which are exportable in multiple User shall able to filter records equipment code wise, date wise, month wise, area wise, activity wise, user wise, Batch number and generate valid reports and enable management to make decision making process.
- You can access your log records from anywhere in the world using a simple web browser still keeping full control over your documents by hosting them on your own server onsite or cloud based This will encourages a standard logbook approach across multi-site operations.
- Keep a record of the Equipment Master’s information, including equipment details and preventative maintenance frequencies.
- Create annual Preventive Maintenance (PM) schedules for all equipment in production blocks and Quality Control (QC) departments, including large machinery and PLCs.
- Schedules can be created, updated, or rejected at any stage in the life cycle of the equipment
- The eCalibration Schedule Management system can give full visibility for usage logs as well as real-time information on the state of equipment and devices.
- When a piece of equipment is out of calibration or has been dumped, the software offers a distinct process flow that a user must It also features a procedure for discarding equipment.
- An overview of the whole instrument’s maintenance records, as well as the calibration dates and activities performed in the process of calibration, will be automatically created.
- Equipment due for Preventive Maintenance can be viewed with just a click of a mouse.
- You can examine ongoing and forthcoming calibration and preventive maintenance routines depending on location and department using the calendar schedule.
- You can effectively track the progress of inquiries for out of calibration (OOC) records and take appropriate remedial action in a timely way.
- A user can specify the calibration intervals (days, months, or years) and how they want the timeframe to be computed. It will automatically generate the calibration target date based on these user preset intervals.
It aids companies in keeping accurate records in accordance with authorized regulations and the SOPs of the organization. The software also allows you to seamlessly examine and validate equipment records.
- Web Based/Cloud Based/ Desktop Supported Technology.
- Pharmaceutical Special Parent- Child Multilevel Table View.
- Control Multilevel Table internal logics and perform appropriate actions.
- Special Report engine server for Generate special pharmaceutical reports.
- Control & Manage multilevel of internal views.
- No requirement of new year process at the end of financial year.
- Comply cGMP, Schedule M and other regulatory requirement.
- Comply software validation requirement.
- Top to Bottom and Bottom to Top information traceability.
- Real time data available any where any time for immediate resolution of any issues.
- System driven activities to make all the activities smoother without missing any of them.
- Equipment management system with maintenance and utilization record.
- Facility to convert reports in to Excel, CSV, PDF, HTML.
- Audit trail available.
- User Management, Session Control
- Document approval / rejection / cancellation system up to multi levels.
- Digital signature on the document to save time and paper.
- Business intelligence tool for analysis available on web / tablet / smart phone etc.
- Less paper, less storage, less people, less space, less effort, less time hence less cost and more profit.
Implementation Process
| Sr.no | Phases | Process | Description |
|---|---|---|---|
| 1 | Phase-1 | Identify | Requirement Gathering, Business Process Mapping |
| 2 | Phase-2 | Analysis | Gap Analysis, Process Design and Approval |
| 3 | Phase-3 | Build & Validation | Development, Testing and Unit Testing |
| 4 | Phase-4 | Deployment | Business Process Installation & Configuration |
| 5 | Phase-5 | Training & Implementation | Data Migration, Attribute of user training, User Practice |
| 6 | Phase-6 | Handover & Support | Golive, Support, Handover & Online Support |

Solution & Support
- B-Line Suite Agile Delivery
- Implementation, Training, Customization & Development
- Data Migration & Third Party Software Integration
- Support -Telephonic, Email, Chat, Video Conference, Onsite
- Premium Support - Assign Dedicated Team Member
Pharma Diagnosis System
Pharmaceutical Diagnosis system is tidily integrated with Production, Operation and Quality test module. It is a
system which helps the user in taking appropriate action in case of any interruption or failure in any process and
test. Tidily linked with eBMR and QMS
B-Line Suite Interface Framework (BIF)

